

The video below shows several different long chained oils, each progressively more viscous. Welcome to the grade 9 chemistry vocabulary list Below you will find a comprehensive list of the key ideas and concepts needed to successfully navigate our chemistry unit. Glycerol, CH 2OHCHOHCH 2OH, is viscous partly because of the length of the chain but also because of the extensive possibilities for hydrogen bonding between the molecules. Conjugate - multiple chemistry definitions, referring to Bronsted acids and bases, a compound formed by combining other compounds, or the overlap of p-orbitals. Fuel oil, lubricating grease, and other long-chain alkane molecules are quite viscous for this reason. This page details why certain liquids flow easily while others are slow as molasses. This is because the molecular chains get tangled up in each other like spaghetti-in order for the liquid to flow, the molecules must first unravel. 2.1: What is Viscosity Viscosity is a fluids resistance to flow. Liquids containing long molecules are invariably very viscous. Honey, mostly glucose and fructose (see image below) is a good example of a liquid which owes its viscosity to hydrogen bonding. Most ordinary liquids have viscosities on the order of 1 to 1000 mPa s, while gases have viscosities on the order of 1 to 10 Pa s. (from the Greek words for heat and power) is the study of heat, work. Chemistry 115 Chapter 1: Chemistry and Measurement What is. The viscosity of water at 20 ☌ is 1.0020 millipascal seconds (which is conveniently close to one by coincidence alone). We begin our study of physical chemistry with thermodynamics. Physical Chemistry Viscosity Measurement of viscosity Ostwald Atmospheric Chemistry Ozone Measurements. Liquids whose molecules are polar or can form hydrogen bonds are usually more viscous than similar nonpolar substances. Viscosity is first and foremost a function of material. These various definitions are why mass transfer often has a reputation with students of. And peanut butter is more viscous than ketchup. Ketchup is more difficult to pour than water, because ketchup is more viscous. The more viscous a substance, the slower it flows. Viscos-ity refers to a fluid’s resistance to flow.
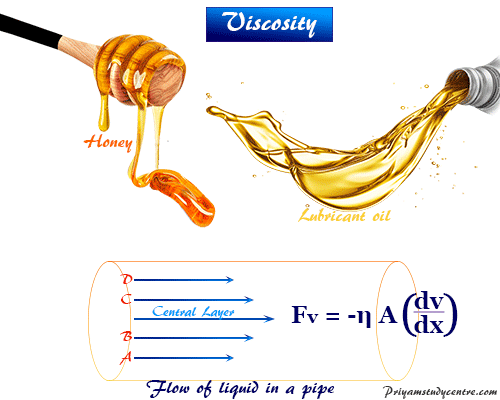
Viscosity is governed by the strength of intermolecular forces and especially by the shapes of the molecules of a liquid. ternatives rivaled only by standard states for chemical potentials. Newton observed that the viscosity of fluids is affected only by temperature. Those like ether or gasoline which flow very readily have low viscosities. Liquids which flow very slowly, like glycerin or honey, have high viscosities. The resistance to such flow is called the viscosity. \)īecause its molecules can slide around each other, a liquid has the ability to flow.


 0 kommentar(er)
0 kommentar(er)
